|
|
 发表于 21-8-2009 05:19 PM
|
显示全部楼层
发表于 21-8-2009 05:19 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 21-8-2009 06:02 PM
|
显示全部楼层
发表于 21-8-2009 06:02 PM
|
显示全部楼层
原帖由 jochen 于 21-8-2009 10:24 AM 发表 
9月前 below USD2 是好价位。。。。
这几天 可以 进场 了。。。。。
yuhoo....
是咯..什么原因那么有信心?? |
|
|
|
|
|
|
|
|
|
|
|
 发表于 21-8-2009 11:45 PM
|
显示全部楼层
发表于 21-8-2009 11:45 PM
|
显示全部楼层
原帖由 napster 于 21-8-2009 11:29 AM 发表 
什么原因让你觉得9月后会起到〉$2?
还有多少个FDA approval?
我倒认为九月份股市大调整
故事大调整。。。
药股 所受的影响不如 金融股。。。
有跟这股的人。。。会知道
7月 挪后 到 9月的opaxio 就快揭晓了。。。
9月前 进是好价为。。。。
个人看法。。。。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 21-8-2009 11:54 PM
|
显示全部楼层
发表于 21-8-2009 11:54 PM
|
显示全部楼层
原帖由 jochen 于 21-8-2009 11:45 PM 发表 
故事大调整。。。
药股 所受的影响不如 金融股。。。
有跟这股的人。。。会知道
7月 挪后 到 9月的opaxio 就快揭晓了。。。
9月前 进是好价为。。。。
个人看法。。。。
上次叶芬说有3个等待FDA,
另外两个大概什么时候可以知道?
so far CTIC 有多少个成功夺得FDA approval?
opaxio的几率高吗?
谢谢 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 22-8-2009 02:00 AM
|
显示全部楼层
发表于 22-8-2009 02:00 AM
|
显示全部楼层
原帖由 napster 于 21-8-2009 11:54 PM 发表 
上次叶芬说有3个等待FDA,
另外两个大概什么时候可以知道?
so far CTIC 有多少个成功夺得FDA approval?
opaxio的几率高吗?
谢谢
opaxio -9月
pixantrone - 如果 下星期 FDA granted "priority review"..今年12月尾 或 明年头就揭晓。。
brostallicin - phase II
http://www.celltherapeutics.com/development
opaxio的几率高吗? 我是当局者, 肯定说高。。。
旁观者 就看个人怎么看。。。。
50-50。。。。赌大小。。。
要就大好。。。要就大坏。。
之前比较危险。。0。30+时。。。随时 BK..
现在似乎比较乐观些。。。。。许多institute...投资者 支持着。。。
叶芬姐 懂得比较多。。看他发的贴。。应该会了解多一些。。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 23-8-2009 11:05 AM
|
显示全部楼层
发表于 23-8-2009 11:05 AM
|
显示全部楼层
CTIC Stock Analysis August 24 2009
CTIC Technical Analysis - The following is Cell Therapeutics ( CTIC ) stock technical analysis for August 24, 2009
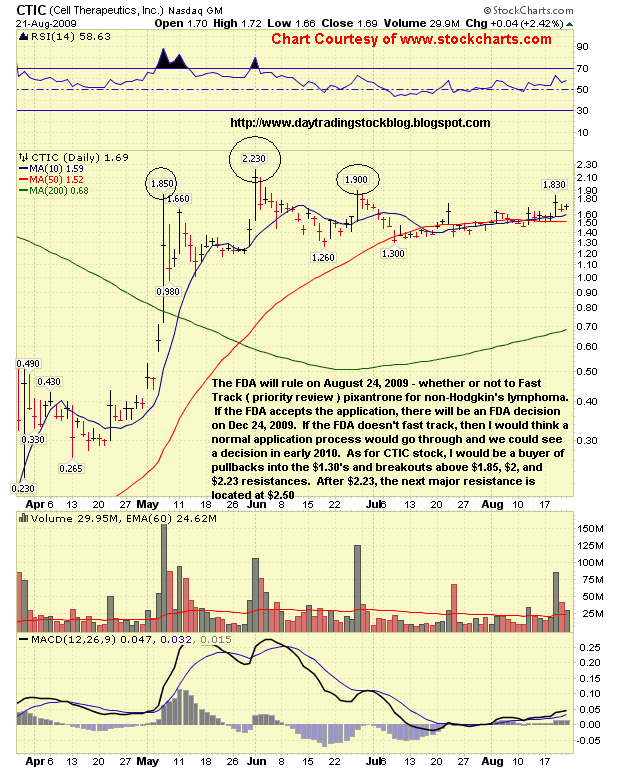
Cell Therapeutics, Inc ( CTIC ) Support & Resistance Levels - 8/24/09
Resistance Levels: $1.85, $1.90, $2, $2.23, $2.50
Support Levels: 1.63, $1.50, $1.46, $1.35
The FDA will rule on August 24, 2009 - whether or not to Fast Track ( priority review ) pixantrone for non-Hodgkin's lymphoma. If the FDA accepts the application, there will be an FDA decision on Dec 24, 2009. If the FDA does not fast track, then I would think a normal application process would go through and we could see a decision in early 2010. As for CTIC stock, I would be a buyer of pullbacks into the $1.30's and breakouts above $1.85, $2, and $2.23 resistances. After $2.23, the next major resistance is located at $2.50. |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 23-8-2009 03:05 PM
|
显示全部楼层
有那么准吗  |
|
|
|
|
|
|
|
|
|
|
|
 发表于 23-8-2009 03:28 PM
|
显示全部楼层
发表于 23-8-2009 03:28 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 23-8-2009 06:12 PM
|
显示全部楼层
发表于 23-8-2009 06:12 PM
|
显示全部楼层
原帖由 joanne27 于 23-8-2009 03:28 PM 发表 
要发就靠ctic lor
我的馬爾代夫之旅就靠她了。。  |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 01:16 PM
|
显示全部楼层
发表于 24-8-2009 01:16 PM
|
显示全部楼层
回复 347# jochen 的帖子
十二月是公布这个吗??
CTIC 將要進軍歐洲市場
張貼 - 三, 2009-07-29 19:02由NAI Editing
CELL THERAPEUTICS INC (NASDAQ TIC) 今天的成交量超過了1800萬股。這家總部設在西雅圖的公司今天宣佈,公司已接到歐洲藥品局(EMEA)的通知,旗下的復發性、侵襲性非何傑金淋巴瘤(NHL)治療藥物pixantrone藥物具備了提交上市許可申請(MAA)的資格。如若得到EMEA的上市批准,就能夠在歐洲市場銷售。CTI已向美國食品和藥物管理局(FDA)提交了pixantrone新藥應用申請(NDA),預計審批結果將於2009年第四季度得到。今天該公司股價上漲了0.08美元,達到了1.5美元。 TIC) 今天的成交量超過了1800萬股。這家總部設在西雅圖的公司今天宣佈,公司已接到歐洲藥品局(EMEA)的通知,旗下的復發性、侵襲性非何傑金淋巴瘤(NHL)治療藥物pixantrone藥物具備了提交上市許可申請(MAA)的資格。如若得到EMEA的上市批准,就能夠在歐洲市場銷售。CTI已向美國食品和藥物管理局(FDA)提交了pixantrone新藥應用申請(NDA),預計審批結果將於2009年第四季度得到。今天該公司股價上漲了0.08美元,達到了1.5美元。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 01:21 PM
|
显示全部楼层
发表于 24-8-2009 01:21 PM
|
显示全部楼层
回复 347# jochen 的帖子
十二月是公布这个吗????
CTIC 將要進軍歐洲市場
張貼 - 三, 2009-07-29 19:02由NAI Editing
CELL THERAPEUTICS INC (NASDAQ TIC) 今天的成交量超過了1800萬股。這家總部設在西雅圖的公司今天宣佈,公司已接到歐洲藥品局(EMEA)的通知,旗下的復發性、侵襲性非何傑金淋巴瘤(NHL)治療藥物pixantrone藥物具備了提交上市許可申請(MAA)的資格。如若得到EMEA的上市批准,就能夠在歐洲市場銷售。CTI已向美國食品和藥物管理局(FDA)提交了pixantrone新藥應用申請(NDA),預計審批結果將於2009年第四季度得到。今天該公司股價上漲了0.08美元,達到了1.5美元。 TIC) 今天的成交量超過了1800萬股。這家總部設在西雅圖的公司今天宣佈,公司已接到歐洲藥品局(EMEA)的通知,旗下的復發性、侵襲性非何傑金淋巴瘤(NHL)治療藥物pixantrone藥物具備了提交上市許可申請(MAA)的資格。如若得到EMEA的上市批准,就能夠在歐洲市場銷售。CTI已向美國食品和藥物管理局(FDA)提交了pixantrone新藥應用申請(NDA),預計審批結果將於2009年第四季度得到。今天該公司股價上漲了0.08美元,達到了1.5美元。 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 02:53 PM
|
显示全部楼层
发表于 24-8-2009 02:53 PM
|
显示全部楼层
好消息就快了......
http://www.marketwatch.com/story/fda-accepts-to-file-cell-therapeutics-new-drug-application-for-pixantrone-2009-08-24
FDA Accepts to File Cell Therapeutics' New Drug Application for Pixantrone
--FDA opts for standard review -- Cell Therapeutics to discuss merits for priority review -- final decision due September 4th
SEATTLE, Aug 24, 2009 /PRNewswire-FirstCall via COMTEX/ -- Cell Therapeutics, Inc. (CTI) (Nasdaq and MTA: CTIC) announced today that the U.S. Food and Drug Administration (FDA) has accepted and has filed for review the Company's New Drug Application (NDA) for pixantrone as treatment for relapsed or refractory aggressive non-Hodgkin's lymphoma (NHL). A Prescription Drug User Fee Act (PDUFA) date will be established by the FDA regarding the review of the pixantrone NDA by September 4th 2009.
"The FDA's acceptance to file our pixantrone NDA represents a significant milestone for CTI and for patients with relapsed and refractory aggressive NHL. We look forward to working with the FDA and their final decision on our request for priority review," noted James Bianco, M.D., Chief Executive Officer of CTI. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 02:54 PM
|
显示全部楼层
发表于 24-8-2009 02:54 PM
|
显示全部楼层
|
卡........................ |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 02:55 PM
|
显示全部楼层
发表于 24-8-2009 02:55 PM
|
显示全部楼层
|
卡...................................2 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 02:55 PM
|
显示全部楼层
发表于 24-8-2009 02:55 PM
|
显示全部楼层
|
卡.................................3 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 02:55 PM
|
显示全部楼层
发表于 24-8-2009 02:55 PM
|
显示全部楼层
|
卡........................4 |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 02:58 PM
|
显示全部楼层
发表于 24-8-2009 02:58 PM
|
显示全部楼层
|
卡...............................?? |
|
|
|
|
|
|
|
|
|
|
|

楼主 |
发表于 24-8-2009 02:58 PM
|
显示全部楼层
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 03:49 PM
|
显示全部楼层
发表于 24-8-2009 03:49 PM
|
显示全部楼层
也可以算是好消息? 延遲 好過 拒絕。
FDA Accepts to File Cell Therapeutics' New Drug Application for Pixantrone
FDA opts for standard review -- Cell Therapeutics to discuss merits for priority review -- final decision due September 4th
* Press Release
* Source: Cell Therapeutics, Inc.
* On Monday August 24, 2009, 1:30 am EDT
*
Companies:
o Cell Therapeutics, Inc.
SEATTLE, Aug. 24 /PRNewswire-FirstCall/ -- Cell Therapeutics, Inc. (CTI) (Nasdaq and MTA: CTIC) announced today that the U.S. Food and Drug Administration (FDA) has accepted and has filed for review the Company's New Drug Application (NDA) for pixantrone as treatment for relapsed or refractory aggressive non-Hodgkin's lymphoma (NHL). A Prescription Drug User Fee Act (PDUFA) date will be established by the FDA regarding the review of the pixantrone NDA by September 4th 2009.
"The FDA's acceptance to file our pixantrone NDA represents a significant milestone for CTI and for patients with relapsed and refractory aggressive NHL. We look forward to working with the FDA and their final decision on our request for priority review," noted James Bianco, M.D., Chief Executive Officer of CTI.
About Pixantrone
Pixantrone (BBR 2778), is a novel topoisomerase II inhibitor with an aza-anthracenedione molecular structure that differentiates it from currently marketed anthracyclines and other related chemotherapy agents. Anthracyclines are the cornerstone therapeutic for the treatment of lymphoma, leukemia, and breast cancer. Although anthracyclines are sufficiently effective to be used as first-line (initial) treatment, anthracyclines cause cumulative heart damage that may result in congestive heart failure many years later. As a result, there is a lifetime limit of anthracycline doses and most patients who previously have been treated with an anthracycline are not able to receive further anthracycline treatment if the patient's disease returns. Pixantrone also can be administered through a peripheral vein rather than a central implanted catheter as required for other drugs in this class. The FDA is required to set an action date for review of an application 74 days after the initial submission of the NDA. CTI expects to receive the action date for this drug candidate from the FDA and a final decision on review status on September 4th, 2009. |
|
|
|
|
|
|
|
|
|
|
|
 发表于 24-8-2009 03:52 PM
|
显示全部楼层
发表于 24-8-2009 03:52 PM
|
显示全部楼层
呵呵 你快我一步。。。
 |
|
|
|
|
|
|
|
|
|
| |
 本周最热论坛帖子 本周最热论坛帖子
|